Abstract
Background: The widespread availability of molecular testing for JAK2 mutations has facilitated the diagnosis of polycythemia vera (PV) but also raises the concern of test overutilization in patients referred for elevated hemoglobin. At our institution, we have observed increased molecular testing in these patients with declining rates of JAK2 mutation positivity, suggesting that a prediction rule could be useful to guide such testing. In this study, we report the derivation and validation of a simple rule using complete blood count (CBC) parameters to predict the likelihood of having a JAK2 mutation in patients referred for elevated hemoglobin.
Methods: We examined all patients with elevated hemoglobin (≥160 g/L for women, or ≥165 g/L for men), who underwent JAK2 mutation testing using the Next-Generation Sequencing (NGS)-based Oncomine Myeloid Research Assay (ThermoFisher Scientific, MA, USA), between 2018 and 2021 at the London Health Sciences Centre in Ontario, Canada. We extracted data including age and sex as well as CBC parameters at the time of testing, including hemoglobin, hematocrit, erythrocytes, leukocytes, neutrophils, platelets and mean corpuscular volume. All CBCs were performed on a Sysmex XN Analyzer (Sysmex Corporation, Japan). In the derivation cohort, JAK2-positive and -negative groups were compared using Student's t-tests or c 2 tests, as appropriate. We dichotomized potentially significant continuous variables at an optimal cut-off point using receiving operating characteristic curves. Potentially significant predictors were evaluated using multiple variable stepwise logistic regression analysis with JAK2 positivity as the dependent variable. The model was evaluated using Hosmer-Lemeshow tests and pseudo-R2 measures. A dichotomous score was derived based on the presence or absence of significant variables and subsequently evaluated and internally validated using logistic regression and c 2 tests using non-parametric bootstrapping with 1000 samples. The model was subsequently validated in the second cohort.
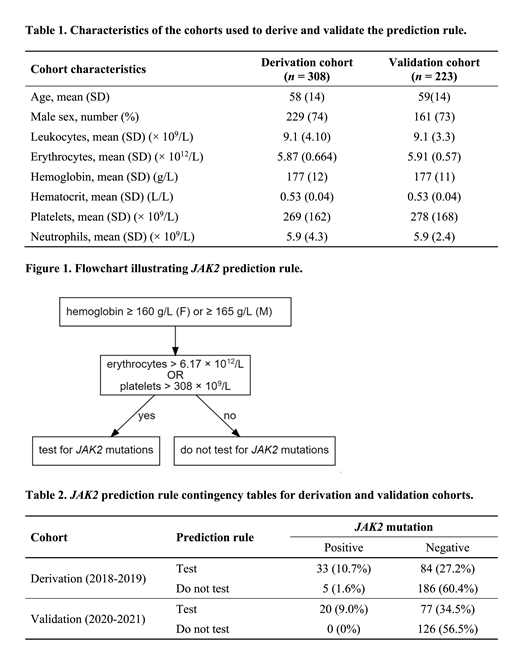
Results: The derivation cohort included 308 patients tested between January 9, 2018 and December 19, 2019, and the validation cohort included 223 patients tested between January 7, 2020 and May 12, 2021. The characteristics of both cohorts are shown in Table 1. The final model included platelets above the upper quintile (308 × 10 9/L) and erythrocytes above the upper quartile (6.17 × 10 12/L) and a score of one was assigned to patients with either of these characteristics. The odds ratio for JAK2 positivity in patients with a score of 1 was 14.6 (95% CI 5.5-38.8) compared to those with a score of 0. The model had a sensitivity of 87.8% and a negative predictive value of 97.4% in the derivation cohort, and of 100% for both in the validation cohort. The percentage of JAK2 positive patients in patients with a score of 1 was 28%. The percent of false negatives was 2.6% (95% CI 1.1-6.0) and 0 (95% CI 0-2.8) in the derivation and validation cohorts, respectively. The use of this rule to guide molecular testing would have resulted in approximately 60% fewer tests.
Conclusion: We developed and validated a simple rule to predict the likelihood of JAK2 mutation positivity in patients with a hemoglobin of 160 or higher, based on CBC parameters with a high negative predictive value (Figure 1). If implemented, this prediction rule could result in a significant reduction in molecular testing avoiding 60% or approximately 100 tests per year at our institution. This approach would be particularly beneficial for broader health system management of hematological malignancies, facilitating the reallocation of resources to emerging higher-yield molecular diagnostic investigation (Kawata et al., BJH 2021).
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal